Nurix Therapeutics’ NX-5948 shows promise in Waldenstrom’s macroglobulinaemia trial
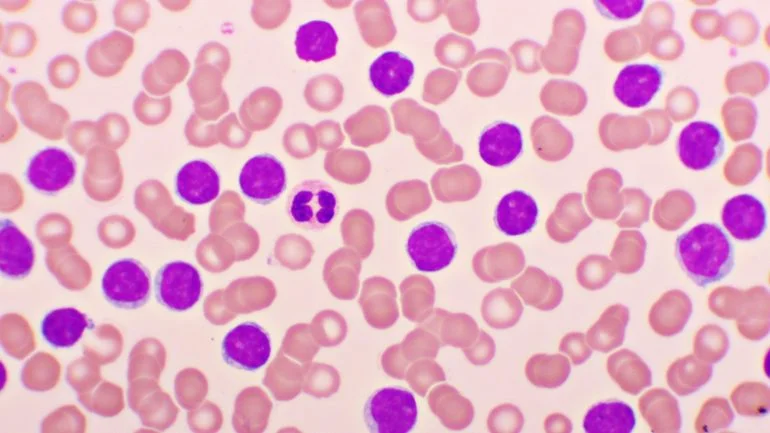
US-based biopharmaceutical company Nurix Therapeutics has reported positive clinical data from its ongoing Phase Ia/Ib trial of NX-5948, a BTK degrader designed to treat relapsed/refractory Waldenstrom’s macroglobulinaemia (WM).
The findings were shared at the 12th International Workshop on Waldenstrom’s Macroglobulinemia in Prague, Czech Republic, which was held from 17 to 19 October.
WM is a rare non-Hodgkin’s lymphoma marked by abnormal lymphocytes and monoclonal immunoglobulin M (IgM) production.
As of 17 April, the trial data showed NX-5948’s safety across all dosages from 50mg to 600mg administered orally daily.
In the trial, the therapy showed an objective response in seven out of nine evaluable patients (77.8%), with two patients showing stable disease.
See Also:MSD mulls 2025/26 launch .
Seven responses were recorded at the first eight-week assessment while five patients are still undergoing treatment, with two of these patients having been treated for more than a year.
Event : International Top Pharmaceutical Awards
Visit: toppharmaceutical.org
Registration: https://
For Enquiries : pharmaquerys123@gmail.com
Get Connected Here
------------------------------
------------------------------
Pinterest: pinterest.com/
Twitter: x.com/TopPharmaaward
Instagram: instagram.com/
youtube: youtube.com/channel/

Comments
Post a Comment